Title
Extending and controlling lymphocyte functionalities by engineered cytokines
Research Area
Biochemistry, Cell Biology, Immunology
Project Summary
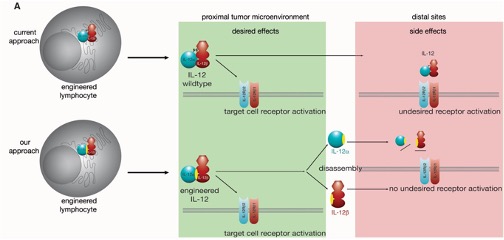
Lymphocytes critically depend on cytokines to regulate and control their diverse biological functions. These allow lymphocytes to be activated when needed, remain active even under adverse conditions and control their activation to maintain immune homeostasis and protect the host. Cytokine engineering thus provides many opportunities for current major challenges in adoptive immunotherapy. This project will seize these opportunities by using rational cytokine and receptor engineering.
Our project will contribute to improving lymphocyte functionalities via advanced molecular engineering approaches. We will closely collaborate with more clinical groups within this consortium to translate our approaches into the diverse biomedically relevant systems available within this CRC/TRR. In addition, we will support other groups within this CRC/TRR with our expertise in protein biogenesis and engineering.
Project-Related Publications
Bohnacker, S., Hildenbrand, K., Aschenbrenner, I., Müller, S.I., Esser-von Bieren, J.*, and Feige, M.J.* (2020). Influence of glycosylation on IL-12 family cytokine biogenesis and function. Mol Immunol 126, 120-128.
Meier, S., Bohnacker, S., Klose, C.J., Lopez, A., Choe, C.A., Schmid, P.W.N., Bloemeke, N., Rührnößl, F., Haslbeck, M., Esser-von Bieren, J., Sattler, M., Huang, P.S., and Feige M.J.* (2019). The molecular basis of chaperone-mediated inter- leukin 23 assembly control. Nat Commun 10, 4121.
Müller, S.I., Aschenbrenner, I., Zacharias, M.*, and Feige, M.J.* (2019). An interspecies analysis reveals molecular construction principles of interleukin 27. J Mol Biol 231, 4383-4393.
Müller, S.I., Friedl, A., Aschenbrenner, I., Esser-von Bieren, J., Zacharias, M., Devergne, O.*, and Feige, M.J.* (2019). A folding switch regulates interleukin 27 biogenesis and secretion of its a-subunit as a cytokine. Proc Natl Acad Sci U S A 116, 1585-1590.
Reitberger, S., Haimerl, P., Aschenbrenner, I., Esser-von Bieren, J., and Feige, M.J.* (2017). Assembly-induced folding regulates interleukin 12 biogenesis and secretion. J Biol Chem 292, 8073-8081.
Feige, M.J.*, Behnke, J., Mittag, T., and Hendershot, L.M.* (2015). Dimerization-dependent folding underlies assembly control of the clonotypic aßT cell receptor chains. J Biol Chem 290, 26821-26831.
Feige, M.J.*, Gräwert, M.A., Marcinowski, M., Hennig, J., Behnke, J., Ausländer, D., Herold, E.M., Peschek, J., Castro, C.D., Flajnik, M.F., Hendershot, L.M., Sattler, M., Groll, M., and Buchner, J. (2014). The structural analysis of shark IgNAR antibodies reveals evolutionary principles of immunoglobulins. Proc Natl Acad Sci U S A 111, 8155-8160.
Feige, M.J.*, and Hendershot, L.M.* (2013). Quality control of integral membrane proteins by assembly-dependent mem- brane integration. Mol Cell 51, 297-309.
Feige, M.J., Groscurth, S., Marcinowski, M., Shimizu, Y., Kessler, H., Hendershot, L.M., and Buchner, J.* (2009). An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol Cell 34, 569-579.
Feige, M.J., Groscurth, S., Marcinowski, M., Yew, Z.T., Truffault, V., Paci, E., Kessler, H., and Buchner, J.* (2008). The structure of a folding intermediate provides insight into differences in immunoglobulin amyloidogenicity. Proc Natl Acad Sci U S A 105, 13373-13378.
*corresponding authors