Title
One CAR – multiple antigens: Universal CAR-T that are chemically programmed to recognize tumors
Research Area
Hematology, Oncology
Project Summary
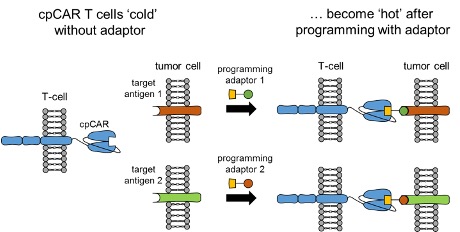
This project aims to overcome the ‘one CAR – one antigen axiome’ and will explore a novel chemically programmable CAR (cpCAR) that can be redirected to target multiple tumor antigens. Conventional CARs recognize a single tumor antigen, which is an impediment for targeting heterogenous tumor cell populations, and fosters tumor escape due to antigen loss. To overcome this limitation, we will employ a cpCAR that allows targeting multiple antigens simultaneously or sequentially, and provides a versatile ‘hub’ technology. The binding domain of this cpCAR is derived from the chemically reactive humanized monoclonal antibody (mAb) 38C2 that comprises a reactive lysine residue to form a covalent bond with diketone (DK)-derivatives of synthetic peptidomimetic compounds.
In Aim 1 of this project, we will program the cpCAR with a cyclic RGD-diketone (cRGD-DK) compound to redirect the specificity of T cells to integrin αVβ3, and compare antigen recognition, signaling and ensuing anti-tumor function to T cells that express a ‘conventional’ integrin αVβ3-specific CAR that is equipped with a singlechain variable fragment (scFv) derived from mAb LM609. Expression of integrin αVβ3 has been shown on primary and metastatic tumor cells in several entities (e.g. melanoma, glioblastoma, breast, lung, pancreatic and prostate cancer). Notably, in these cancers, integrin αVβ3 is associated with epithelial-to-mesenchymal transition (EMT) and drug resistance. In addition, integrin αVβ3 is found on tumor stroma and vasculature. In Aim 2, we will therefore use the cpCAR as a co-receptor to provide additional costimulatory signals in trans to a primary tumor-reactive CAR, and will analyze to what extent engagement of integrin αVβ3 augments CAR-T cell function. In Aim 3 we will pursue novel strategies to augment CAR proximal signaling, based on our preliminary work showing that current CAR designs poorly engage ZAP-70 and other members of the signaling cascade in human T cells.
Project-Related Publications
Hudecek M, Lupo-Stanghellini M-T, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR (2013). Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 19(12):3153–64.
Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR (2015). The non- signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 2015 Feb;3(2):125-35.
Monjezi R, Miskey C, Gogishvili T, Schleef M, Schmeer M, Einsele H, Ivics Z, Hudecek M (2017). Enhanced CAR T cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia 31(1):186–194.
Wallstabe L, Mades A, Frenz S, Einsele H, Rader C, Hudecek M (2018). CAR T cells targeting αVβ3 integrin are effective against advanced cancer in preclinical models. Adv. Cell Gene Ther. 1(2):e11.
Rydzek J, Nerreter T, Peng H, Jutz S, Leitner J, Steinberger P, Einsele H, Rader C, Hudecek M (2019). Chimeric Antigen Receptor Library Screening Using a Novel NF-κB/NFAT Reporter Cell Platform. Molecular Therapy. 27(2):287–299.
Qi J, Tsuji K, Hymel D, Burke TR, Hudecek M, Rader C, Peng H (2020). Chemically programmable and switchable CAR-T therapy. Angew. Chem. Int. Ed. Engl. 59(29):12178-12185.
Nerreter T, Letschert S, Götz R, Doose S, Danhof S, Einsele H, Sauer M, Hudecek M (2019). Super-Resolution Microscopy Reveals Ultra-Low CD19 Expression on Myeloma Cells That Triggers Elimination by CD19 CAR-T. Nature Communications 10(1):3137.
Querques I, Mades A, Zuliani C, Miskey C, Alb M, Grueso E, Machwirth M, Rausch T, Ivics Z, Hudecek M*, Barabas O* (*shared senior authorship) (2019). A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nature Biotechnology 37:1502-12.
Gudipati V, Rydzek J, Doel-Perez I, Dos Reis Gonçalves V, Scharf L, Königsberger S, Lobner E, Kunert R, Einsele H, Stockinger H, Hudecek M*, Huppa J B* (*shared senior authorship) (2020). Inefficient CAR-proximal signaling blunts antigen sensitivity. Nature Immunology 21:848-856.
Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek M (2019). The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR-T cells. Science Trranslational Medicine 11(499): eaau5907.