Title
Engineering CAR-T cells against neuroblastoma with synthetic notch receptors to increase tumor-specificity and endogenous immune activation
Research Area
CAR-T cell therapy
Project Summary
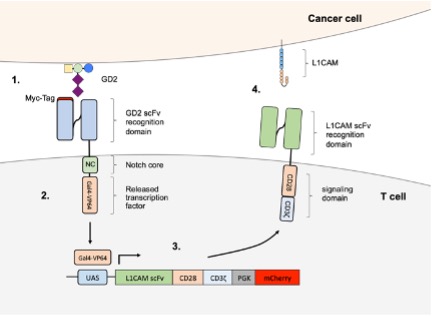
Adoptive T cell therapies are one of the most promising therapeutic approaches to cure cancer, but targeting solid cancers with chimeric antigen receptor (CAR-) T cells remains challenging. A lot of effort goes into lymphocyte engineering with the goal to improve CAR-T cell therapy by increasing CAR-T cell effector function and persistence in an immunosuppressive tumor microenvironment. With enhanced efficacy, the risk increases for on-target, off-tumor toxicity and tumor escape via antigen loss or downregulation. CRISPR/Cas9 geneediting technology makes elaborate lymphocyte engineering possible, but the risk rises that the recipient’s immune system could reject CAR-T cells the more elaborately they are engineered.
Project-Related Publications
Künkele, A., Johnson, A.J., Rolczynski, L.S., Chang, C.A., Hoglund, V., Kelly-Spratt, K.S., Jensen, M.C et al: Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenu- ated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol Res 2015; 3, 368-379
Künkele, A., Taraseviciute, A., Finn, L.S., Johnson, A.J., Berger, C., Finney, O., Chang, C.A., Rolczynski, L.S., Brown, C., Mgebroff, S., Berger, M., Park, J.R., Jensen, M.C.: Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin Cancer Res 2017; 23, 466-477
Künkele, A., Brown, C., Beebe, A., Mgebroff, S., Johnson, A.J., Taraseviciute, A., Rolczynski, L.S., Chang, C.A., Finney, O., Park, J.R., Jensen, M.C.: Manufacture of chimeric antigen receptor T cells from mobilized cryo- preserved peripheral blood stem cell units depends on monocyte depletion. Biol Blood Marrow Transplant 2019; 25:223-232
Andersch, L., Radke, J., Klaus, A., Schwiebert, S., Winkler, A., Schumann, E., Grunewald, L., Zirngibl, F., Flem- mig, C., Jensen, M.C., Rossig, C., Joussen, A., Henssen, A., Eggert, A., Schulte, J.H., Künkele A: CD171- and GD2-specific CAR T cells potently target retinoblastoma cells in preclinical in vitro testing. BMC Cancer 2019; 19, 895
Ali, S., Toews, K., Schwiebert, S., Klaus, A., Winkler, A., Grunewald, L., Oevermann, L., Deubzer, H.E., Tüns, A., Jensen, M.C., Henssen, A.G., Eggert, A., Schulte, J.H., Schwich, E., Rebmann, V., Schramm, A., Künkele A: Tumor-derived extracellular vesicles impair CD171-specific CD4+ CAR T cell efficacy. Front Immunol 2020; 11:531
Toews, K., Grunewald, L., Schwiebert, S., Klaus, A., Winkler, A., Ali, S., Zirngibl, F., Astrahantseff, K., Wagner, D.L., Henssen, A.G., Deubzer, H.E., Schulte, J.H., Ochsenreither, S., Eggert, A., Künkele A: Central memory phenotype drives success of checkpoint inhibition in combination with CAR T cells. Mol Carcinog 2020; 59:p.724- 735