Title
Engineering of CAR-T cells that overcome MYC-dependent immunosuppression in a genetically engineered mouse model of pancreatic cancer
Research Area
Hematology, Oncology, Transfusion Medicine (205-14); Gastroenterology, Metabolism (205-15)
Project Summary
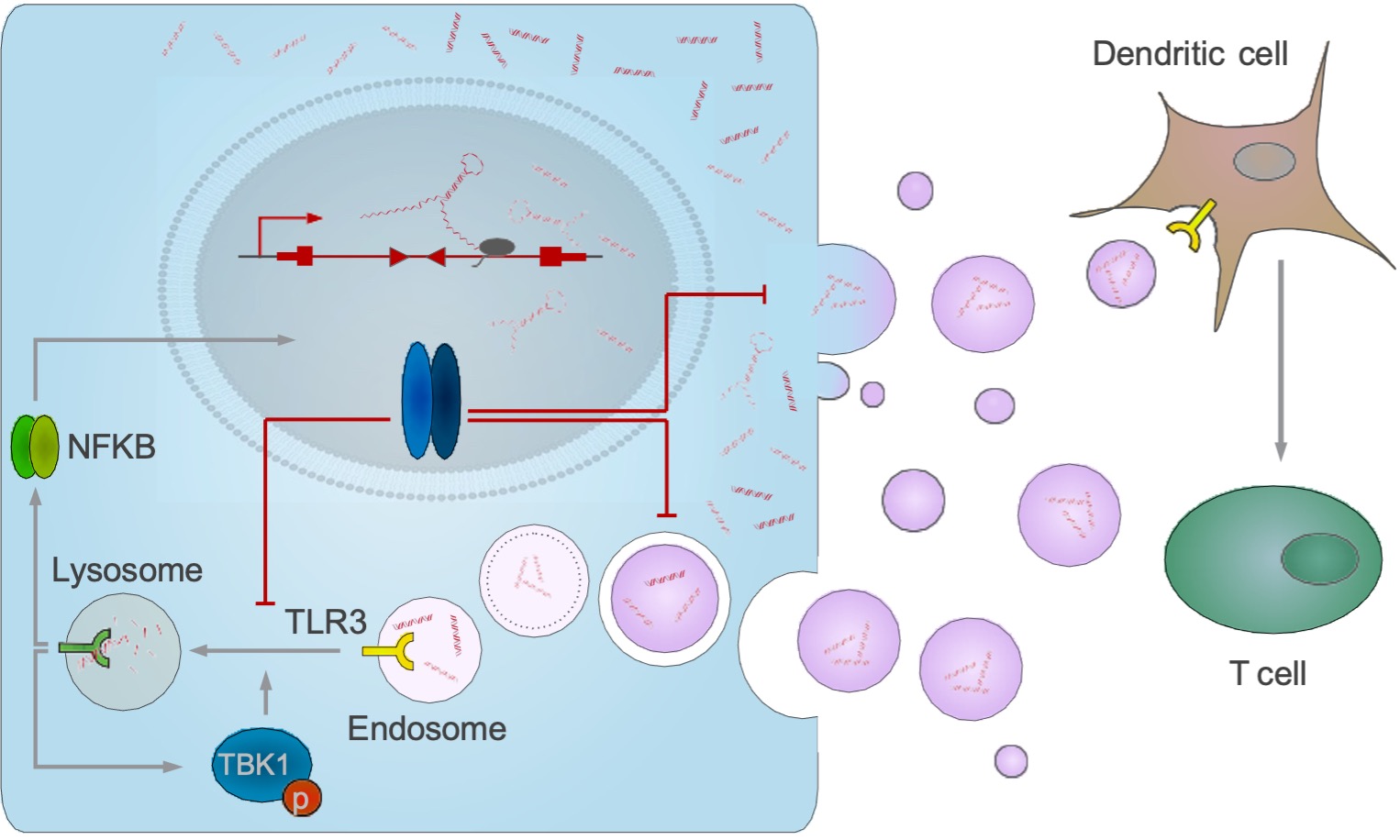
Amplification or increased expression of the MYC proto-oncogene or one of its paralogues, MYCN and MYCL, is a hallmark of the majority of all human tumors and many tumors depend on elevated MYC expression throughout their lifetime. Recent evidence argues that a major reason for this dependency is the ability of MYC to enable tumor cells to escape immune surveillance. MYC compromises the T cell response through several mechanisms including (1) direct induction of the expression of the immune checkpoint programmed deathligand 1 on tumor cells, (2) suppression of a broad range of cytokines and (3) reduction of exosomal secretion of intron-derived dsRNA from tumor cells.
Genetically modified T cells expressing a chimeric antigen receptor (CAR) directed against tumor surface antigens are a promising concept for the treatment of cancer. However, the therapeutic efficiency of CAR-T cells is often compromised in the tumor niche. In this project, we will dissect how MYC-dependent tumorintrinsic factors impair the functionality of ROR1-directed CAR-T cells and we will explore T cell engineering strategies to render the adoptively transferred lymphocytes at least temporarily unresponsive to inhibitory factors coming from the tumor (microenvironment). To this end, we will study a mouse model of pancreatic carcinoma (PDAC) driven by a mutated KRAS oncogene and a mutated p53 gene. This model (LSL-KrasG12D/+;LSL- Trp53R172H/+;Pdx-1- Cre (“KPC”)) is poorly immunogenic and faithfully mimics the human disease. We have used several strategies to inducibly deplete or delete the endogenous MYC gene. These strategies lead to a slow-down of proliferation in culture, and to an immune cell-mediated tumor regression and greatly extended survival in vivo. Engineering strategies will include (1) the protection of CAR T cells from known factors of MYC-driven immunosuppression (PD-L1, TGF-β), (2) the augmentation of T cell susceptibility to microvesicular dsRNA and (3) the exploration of further beneficial T cell modifications as evaluable in functional CRISPR/Cas9 screens to define relevant gene products in T cells to overcome MYC-driven immunosuppression.
Project-Related Publications
Rodríguez-Lobato LG, Ganzetti M, Fernandéz de Larrea C, Hudecek M, Einsele H, Danhof S (2020). CAR T-Cells in Multiple Myeloma: State of the Art and Future directions. Front. Oncol. 10:1243
Haikala H, Anttila J, Marques E, Raatikainen T, Ilander M, Hakanen H, Ala-Hongisto H, Savelius M, Balboa D, von Eyss B, Eskelinen V, Munne P, Nieminen A, Otonkoski T, Schüler J, Laajala T, Aittokallio T, Sihto H, Mattson J, Heikkilä P, Leidenius M, Joensuu H, Mustjoki S, Kovanen P, Eilers M, Leverson J, Klefström J. (2019) Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat Commun 10: 620
Herold S, Kalb J, Büchel G, Ade CP, Baluapuri A, Xu J, Koster J, Solvie D, Carstensen A, Klotz C, Rodewald S, Schülein- V.lk C, Dobbelstein M, Wolf E, Molenaar J, Versteeg R, Walz, S, Eilers M (2019). Recruitment of BRCA1 limits MYCN- driven accumulation of stalled RNA Polymerase. Nature 567(7749):545-549
Nerreter T, Letschert S, Götz R, Doose S, Danhof S, Einsele H, Sauer M, Hudecek M (2019). Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun:10(1):3137.
Gogishvili T*, Danhof S*, Prommersberger S, Rydzek J, Schreder M, Brede C, Einsele H, Hudecek M (2017). SLAMF7- CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 130(26):2838- 2847. *These authors contributed equally.
Dejure FR, Royla N, Herold S, Kalb J, Walz S, Ade CP, Mastrobuoni G, Vanselow J, Schlosser A, Wolf E, Kempa S, Eilers M (2017) The 3’-UTR of MYC couples RNA polymerase II function to ribonucleotide levels. EMBO J. 36(13):1854-1868.
Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Guetgemann I, Eilers M, Felsher DW (2016). MYC Regulates the Anti-Tumor Immune Response through CD47 and PD-L1. Science 52(6282):227-31.
von Eyss B, Jaenicke LA, Kortlever RM, Royla N, Wiese KE, Letschert L, McDuffus LA, Sauer M, Rosenwald A, Evan GI, Kempa S, Eilers M (2015) A MYC-driven change in mitochondrial dynamics limits YAP/TAZ function in mammary epithelial cells and breast cancer. Cancer Cell, 28:743-57.
van Riggelen J, Müller J, Otto T, Beuger V, Yetil A, Choi PS, Kosan C, Möröy T, Felsher DW, Eilers M (2010). The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev 24(12):1281-94.
Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Möröy T, Bartek J, Massagué J, Hänel F, Eilers M
(2001). Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol 3(4):392-9.