Title
Super-resolution microscopy to visualize CAR-T cell receptome and function
Research Area
Biophysics (201-02), Method Development (304-01), Biochemistry (201-01), Cell Biology (201-03), Immunology (204-05), Hematology (205-14)
Project Summary
This project aims to visualize how changes in the design and way of introduction of a chimeric antigen receptor (CAR) influence the ability of CAR-modified T cells to detect, interact with and destroy target cells. We will combine rational receptor design and single-molecule sensitive super-resolution microscopy (SRM) methods including dSTORM and lattice light-sheet microscopy to determine absolute antigen thresholds required to initiate the different T cell effector functions (in vitro cytotoxicity, proliferation and cytokine secretion, in vivo antitumor efficacy in e.g. NSG/tumor mouse models).
Project-Related Publications
Sauer M & Heilemann M. Single-molecule localization microscopy in eukaryotes. Chem Rev 2017; 117(11):7478-7509.
Heilemann M, van de Linde S, Schüttpelz M, Kasper R, Seefeldt B, Mukherjee A, Tinnefeld P, Sauer M. Subdiffractionresolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed 2008; 47(33):6172-6176.
Wäldchen, F, Schlegel, J, Götz, R, Luciano, M, Schnermann, M, Doose, S, Sauer, M. Whole-cell imaging of plasma mem brane receptors by 3D lattice light-sheet dSTORM. Nat Commun 2020; 11:887.
Zwettler, F U, Reinhard, S, Gambarotto, D, Bell, T D M, Hamel, V, Guichard, P, Sauer, M. Molecular resolution imaging by post-labeling expansion single-molecule localization microscopy (SMLM). Nat Commun 2020; 11:3388.
Peng H, Nerreter T, Chang J, Qi J, Li X, Karunadharma P, Martinez GJ, Fallahi M, Soden J, Freeth J, Beerli RR, Grawunder U, Hudecek M, Rader C. Mining Naïve Rabbit Antibody Repertoires by Phage Display for Monoclonal Antibodies of Therapeutic Utility. J Mol Biol 2017; 429(19):2954-2973.
Rydzek J, Nerreter T, Peng H, Jutz S, Leitner J, Steinberger P, Einsele H, Rader C, Hudecek M. Chimeric Antigen Receptor Library Screening Using a Novel NF-κB/NFAT Reporter Cell Platform. Mol Ther 2019; 27(2):287-299.
Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, Bonig H, Herr W, Sierra J, Einsele H, Hudecek
M. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018; 32(5):1168-1179.
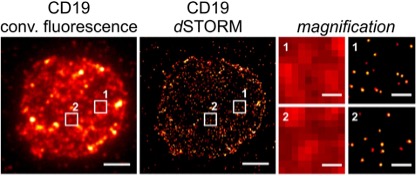
Nerreter T, Letschert S, Götz R, Doose S, Danhof S, Einsele H, Sauer M, Hudedek M. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun 2019; 10(1):3137.
Garcia-Guerrero, E, Götz, R, Doose, S, Sauer, M, Rodriguez-Gil, A, Nerreter, T, Kortüm, M, Pèrez-Simón, J A, Einsele, H, Hudecek, M, Danhof, S. Upregulation of CD38 Expression on Multiple Myeloma Cells by Novel HDAC6 Inhibitors is a Class Effect and Augments the Efficacy of Daratumumab. Leukemia 2020; doi:10.1038/s41375-020-0840-y.
b) Patents
Nerreter T, Letschert S, Einsele H, Sauer M, Hudecek M. CD19CART cells eliminate myeloma cells that express very low levels of CD19. PCT/EP2018/081924.