Title
Engineering CAR-T cells to optimize the treatment of cerebral malignancies
Research Area
Neurooncology, immunotherapy, immunology, in vivo multiphoton imaging, single cell RNA sequencing
Project Summary
In the treatment of brain malignancies, T cells expressing chimeric-antigen-receptors (CARTs) have failed to induce lasting tumor control. To a large extent, this has been attributed to insufficient infiltration, activation and persistence of CARTs within the heterogeneous and immunosuppressive tumor stroma. In this project, we therefore aim to optimize the migratory and tumoricidal properties of CARTs in the treatment of brain tumors. To achieve this goal, we will utilize a set of approaches that investigate the functional and transcriptional properties of CARTs directly ex vivo.
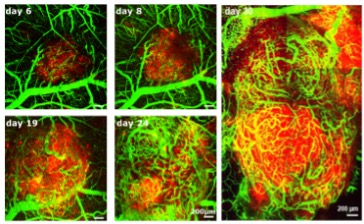
In a second step, we aim to identify and validate candidate genes for successful migratory engineering. We will compare the whole transcriptome of deeply infiltrating CARTs to that of superficially restricted CARTs harvested from the same tumor or tumor entity. To achieve this, CARTs expressing a photo-convertible fluorophore will be used. After photo-activation using in vivo multiphoton confocal laser scanning microscopy (MPLSM), CARTs will be sorted according to their color-coded positional history and will further be processed for RNA sequencing (RNAseq) on bulk and later single-cell level.
Finally, we aim to modify infiltration depth, motility and persistence of individual CARTs in brain tumors by retroviral combinatorial engineering of an additional profile of fluorescently-labeled migration-associated receptors. Using an established optical barcoding approach (Grasmann et al, 2019), the engineered receptor profile of individual tumor infiltrating CARTs can be assessed directly via MPLSM and/or flow-cytometry. Promising receptor combinations will then be tested against standard CART therapy for efficiency and durability of tumor regression.
Project-Related Publications
L. von Baumgarten:
Kienast, Y., von Baumgarten, L., Fuhrmann, M., Klinkert, W.E., Goldbrunner, R., Herms, J., and Winkler, F. (2010). Real- time imaging reveals the single steps of brain metastasis formation. Nat Med 16, 116-122.
von Baumgarten, L., Brucker, D., Tirniceru, A., Kienast, Y., Grau, S., Burgold, S., Herms, J., and Winkler, F. (2011). Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res 17, 6192-6205.
Mulazzani, M., Frassle, S.P., von Mucke-Heim, I., Langer, S., Zhou, X., Ishikawa-Ankerhold, H., Leube, J., Zhang, W., Dotsch, S., Svec, M, Rudelius M, Dreyling M, von Bergwelt-Baildon M, Straube A, Buchholz VR, Busch DH, von Baumgarten L.. (2019). Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice. Proc Natl Acad Sci U S A 116, 24275-24284.
O’Connor, T., Zhou, X., Kosla, J., Adili, A., Garcia Beccaria, M., Kotsiliti, E., Pfister, D., Johlke, A.L., Sinha, A., Sankowski, R.,… von Baumgarten L., Keller U, Heikenwalder M. (2019). Age-Related Gliosis Promotes Central Nervous System Lymphoma through CCL19-Mediated Tumor Cell Retention. Cancer Cell 36, 250-267 e259.
Zhou, X., Mulazzani, M., von Mucke-Heim, I.A., Langer, S., Zhang, W., Ishikawa-Ankerhold, H., Dreyling, M., Straube, A., and von Baumgarten, L. (2020). The Role of BAFF-R Signaling in the Growth of Primary Central Nervous System Lymphoma. Front Oncol 10, 682.
V.R. Buchholz:
Buchholz, V.R., Flossdorf, M., Hensel, I., Kretschmer, L., Weissbrich, B., Graf, P., Verschoor, A., Schiemann, M., Hofer, T., and Busch, D.H. (2013a). Disparate individual fates compose robust CD8+ T cell immunity. Science 340, 630-635.
Cho, Y.L., Flossdorf, M., Kretschmer, L., Hofer, T., Busch, D.H., and Buchholz, V.R. (2017). TCR Signal Quality Modulates Fate Decisions of Single CD4(+) T Cells in a Probabilistic Manner. Cell Rep 20, 806-818.
Graef, P., Buchholz, V.R., Stemberger, C., Flossdorf, M., Henkel, L., Schiemann, M., Drexler, I., Hofer, T., Riddell, S.R., and Busch, D.H. (2014). Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 41, 116-126.
Grassmann, S., Pachmayr, L.O., Leube, J., Mihatsch, L., Andrae, I., Flommersfeld, S., Oduro, J., Cicin-Sain, L., Schiemann, M., Flossdorf, M., and Buchholz, V.R. (2019). Distinct Surface Expression of Activating Receptor Ly49H Drives Differential Expansion of NK Cell Clones upon Murine Cytomegalovirus Infection. Immunity 50, 1391-1400 e1394.
Grassmann, S., Mihatsch, L., Mir, J., Kazeroonian, A., Rahimi, R., Flommersfeld, S., Schober, K., Hensel, I., Leube, J., Pachmayr, L.O., Kretschmer, L., Zhang, Q., Jolly, A., Chaudhry, MZ., Schiemann, M., Cicin-Sain, L., Höfer, T., Busch, D.H., Flossdorf, M. and Buchholz, V.R. (2020). Early emergence of T central memory precursors programs clonal dominance during chronic viral infection. Nat. Immunol. 170, 2022.