Title
Dissection and correction of human regulatory T cell dysfunctions by CRISPR engineering
Research Area
immunology, genetics
Project Summary
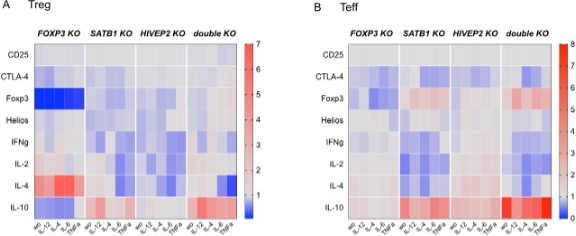
Disturbed balances between regulatory T (Treg) and effector T (Teff) cells predispose to autoimmunity, immunodeficiency, and/or inflammation. Human Treg and Teff functions are attractive targets for different autoimmune diseases. However, our scarce understanding of the complex transcriptional landscape controlling Treg and Teff cell function is hindering the development of novel therapeutic approaches.
In parallel, we will evaluate innovative non-viral CRISPR/Cas9-mediated genome targeting approaches for the correction of human T cell dysfunctions in humanized mouse models. As proof-of-principle, we will evaluate the therapeutic potential of T cell genome editing in a humanized mouse model of CARMIL2 deficiency, a monogenic and life-threatening disease with altered Treg cell differentiation and T cell activation. Safety and sustained functional reconstitution of CARMIL2 deficiency in NSG-HLA-DQ8 mice will be monitored by analyzing (i) CARMIL2 expression, (ii) CD28-mediated signaling, as well as (iii) differentiation and function of Teff and Treg cells. Ultimately, we will evaluate (i) the development of experimental colitis and (ii) safety of genomic engineering by detection of off-target integrations using targeted locus amplification sequencing (Aim2).
Our studies will provide insights on transcriptional regulation of human Treg cell induction and the therapeutic potential of CRISPR/Cas9-mediated engineering of human T cell defects.
Project-Related Publications
Schumann K.*,#, Raju S.S.*, Lauber M., Kolb S., Shifrut E., Cortez J.T., Skartsis N., Nguyen V.Q., Woo J.M., Roth T.L., Yu R., Nguyen M.L.T., Simeonov D.R., Nguyen D.N., Targ S., Gate R.E., Tang Q., Bluestone J.A., Spitzer M.H., Ye C.J., Marson A.#. (2020) Functional CRISPR dissection of gene networks controlling human regulatory T cell identity. Nat Immunol. 21, 1456-1466.
Lowe M.M., Boothby I., Clancy S., Ahn R.S., Liao W., Nguyen D.N., Schumann K., Marson A., Mahuron K.M., Kingsbury G.A., Liu Z., Munoz Sandoval P., Rodriguez R.S., Pauli M.L., Taravati K., Arron S.T., Neuhaus I.M., Harris H.W., Kim E.Al, Shin U.S., Krummel M.F., Daud A., Scharschmidt T.C., Rosenblum M.D. (2019) Regulatory T cells use arginase 2 to enhance their metabolic fitness in tissues. JCI Insight. 19, 4(24).
Rupp L.J.*, Schumann K.*, Roybal K.T., Gate R.E., Ye J.Y., Lim W.A., Marson A. (2017). CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 7, 37.
Hultquist J.F.*, Schumann K.*, Woo J.M., Manganaro L., Doudna J.A., Simon V., Krogan N.J., Marson A. (2016) A Cas9 ribonucleoprotein platform for functional genetic studies of HIV-Host interactions in primary human T Cells. Cell Reports 17, 1438-1452.
Schumann K.*, Lin S.*, Boyer E., Simeonov D.R., Subramaniam M., Gate R.E., Haliburton G.D.E., Ye C.J., Bluestone J.A., Doudna J.A., Marson A. (2015). Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. PNAS 112, 10437-4.2
Goettel J.A.*, Kotlarz D.*, Emani R., Canavan J.B., Konnikova L., Illig D., Frei S.M., Field M., Kowalik M., Peng K., Grin- gauz J., Mitsialis V., Wall S.M., Tsou A., Griffith A.E., Huang Y., Friedman J.R., Towne J.E., Plevy S.E., O’Hara Hall A., Snapper S.B. (2019). Low-Dose Interleukin-2 Ameliorates Colitis in a Preclinical Humanized Mouse Model. Cell Mol Gastroenterol Hepatol. 8, 193-195.
Li Y., Führer M., Bahrami E., Socha P., Klaudel-Dreszler M., Bouzidi A., Liu Y., Lehle A.S., Magg T., Hollizeck S., Rohlfs M., Conca R., Field M., Warner N., Mordechai S., Shteyer E., Turner D., Boukari R., Belbouab R., Walz C., Gaidt M.M., Hornung V., Baumann B., Pannicke U., Al Idrissi E., Ali Alghamdi H., Sepulveda F.E., Gil M., de Saint Basile G., Hönig M., Koletzko S., Muise A.M., Snapper S.B., Schwarz K.*, Klein C.*, Kotlarz D*,#. (2019). Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc Natl Acad Sci U S A. 116, 970-975.
Lehle A.S., Farin H.F., Marquardt B., Michels B.E., Magg T., Li Y., Liu Y., Ghalandary M., Lammens K., Hollizeck S., Rohlfs M., Hauck F., Conca R., Walz C., Weiss B., Lev A., Simon A.J., Groß O., Gaidt M.M., Hornung V., Clevers H., Yazbeck
N., Hanna-Wakim R., Shouval D.S., Warner N., Somech R., Muise A.M., Snapper S.B., Bufler P., Koletzko S., Klein C.*, Kotlarz D.*,# (2019). Intestinal Inflammation and Dysregulated Immunity in Patients With Inherited Caspase-8 Deficiency. Gastroenterology 156, 275-278.
Kotlarz D.*, Marquardt B.*, Barøy T., Lee W.S., Konnikova L., Hollizeck S., Magg T., Lehle A.S., Walz C., Borggraefe I., Hauck F, Bufler P., Conca R., Wall S.M., Schumacher E.M., Misceo D., Frengen E., Bentsen B.S., Uhlig H.H., Hopfner K.P., Muise A.M., Snapper S.B., Strømme P, Klein C. (2018). Human TGF-β1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet. 50, 344-348.
Glocker E.O.*, Kotlarz D.*, Boztug K., Gertz E.M., Schäffer A.A., Noyan F., Perro M., Diestelhorst J., Allroth A., Murugan D., Hätscher N., Pfeifer D., Sykora K.W., Sauer M., Kreipe H., Lacher M., Nustede R., Woellner C., Baumann U., Salzer U., Koletzko S., Shah N., Segal A.W., Sauerbrey A., Buderus S., Snapper S.B., Grimbacher B., Klein C. (2009). Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361, 2033-45.